ISO/IEC 17025
Accreditation from UKAIB Accreditation Registrars
Raise your standards for testing and calibration by gaining ISO/IEC 17025 Accreditation for your laboratories.
Different testing laboratories often produce somewhat different test results for a given same sample due to a variation in staff competency, equipment or tool used for testing, technologies they operate in the labs, and Standard Operating Procedures (SOPs) followed. These variations are due to the use of uncalibrated and unmaintained instruments to updated technologies and techniques, and the competence level of technicians conducting a particular test. Another reason for this variation is often done by manipulating sampling procedures and test results. Well, there can be countless factors and reasons contributing to the inconsistency and inaccuracy of test results and the decisions derived from them! So, to maintain the consistency in the report and test performed; ISO/IEC 17025 accreditation came into force to demonstrate worldwide accepted accurate results.
ISO/IEC 17025 Accreditation is a company-level accreditation published by International Organization for Standardization in cooperation with International Electrotechnical Commission. ISO/IEC 17025 provides “General requirements for the competence of testing and calibration laboratories”. The main motto of this accreditation is to offer quality and improve the processes within laboratories. The standard contains two main sections: 1. Management requirements and, 2. Technical requirements.
Management requirements are primarily related to the operation and effectiveness of the quality management system within the laboratory and technical requirements are primarily related to the competence of staff and calibration of equipment and tools used in the laboratories. The standard also gives requirements related to quality management such as document control and corrective action required to implement if the case arises.
ISO 17025:2017 is the main ISO standard used by third-party laboratories to get their lab’s international identity. In most cases, suppliers and regulators will not accept their test results from a laboratory that does not hold accreditation. Thus, ISO/IEC 17025 is by far for the laboratory to establish competence and quality for their testing and calibration. It also facilitates cooperation between testing laboratories and other bodies by establishing wider acceptance of results. Test reports and Certificates of Analysis (CoA) can be accepted by organizations present in different countries without the need for further testing if your laboratory is accreditated by ISO/IEC 17025 standard, which leads to better international trade—quality as per new technology and techniques to cater to customer requirements and needs.
ISO 13485 requirements cover-up all the Quality Policy, Quality Objectives, and Quality Manual for implementing the Quality Management System. Adopting ISO 13485 provides a practical foundation for manufacturers to address the EU Medical Device Directive (MDD), the EU Medical Device Regulation (MDR), and other regulations, as well as demonstrating a commitment to the safety and quality of medical devices for their users.
A Certification Body applying for ISO 13485 accreditation must conform to ISO/IEC 17021 and other additional International requirements as detailed in Specific Requirements for Accreditation for QMS-MD Scheme.
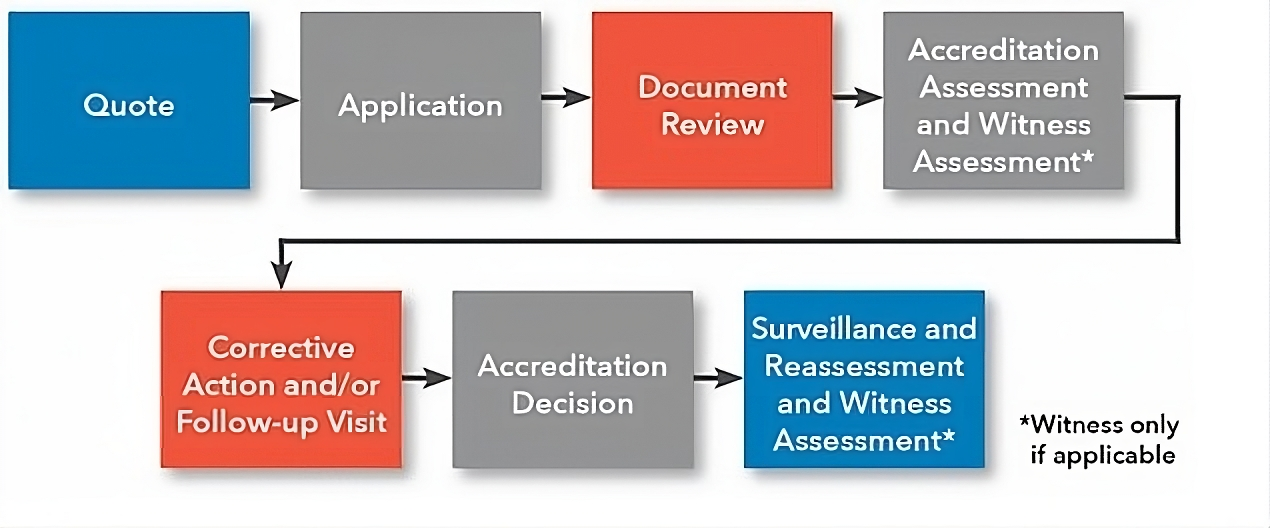
Please refer to the information about the accreditation process
UKAIB Accreditation for Inspection Agencies”
- Demonstrates compliance with ISO 22000 Accreditation.
- UKAIB offers prompt, personal service, including rapid scheduling of assessments to meet the needs of inspection agencies.
- Accreditation serves as an internationally recognized “stamp of approval” for industry and regulators.
- Accreditation increases the recognition and acceptance of inspection reports across domestic and national borders.
- Accreditation helps to reduce costs for manufacturers and exporters by reducing or eliminating the need for re-inspecting in another economy.

Why do you need to be ISO 17020 Accredited?
To take your products/services in the international market your organization needs to comply with the regional and international conformity of the standards.
- By Micro-level survey for every inspection parameter
- Conducting Standard Operating Procedures (SOPs) for inspection
- Formats to establish the objective evidence
- Witnessing/demonstration of inspection activities to build confidence
ISO/IEC 17020:2012 is based on a detailed study of all activities which add value to your organization by making assured that your goods/services are what they claim to be and do not include any fraudulent. In this globalized world with a thick and long supply chain in business and other activities, it becomes rigid to trace the originality of the goods and provide transparency in the procedure. Achieving ISO 17020 Accreditation Certificate helps your business to be a step ahead of your competitor with the assurance of quality and authenticity for your operations.
The ISO 17020 certificate is issued for 2 years with renewable. The Certificate is valid for 2 years from the date of issue and requires a yearly audit to verify compliance of the standard. Upon completion of 2 years cycle of the certificate validity, there is renewal audit will be conducted without any periodic surveillance assessment.
People Who Love Our Place
UKAIB Accreditation Forum Limited has pioneered novel approaches to accreditation that permit benchmarking via measurable evaluation of conformity assessment body (CAB), performance.




