GMP
Accreditation for GMP (Good Manufacturing Practice)
UKAIB accredits Certification Bodies for compliance against GMP through verification of their existing ISO 17021 system. The accreditation is done against Scheme No- 22001.
GMP Accreditation for a Certification body (CBs) helps them to audit and provide accreditated Certification to their Clients.
GMP is the Good Manufacturing Practice also referred to as cGMP helps to that ensures that medicinal products are consistently produced and controlled to the quality standards. It helps companies to minimize or eliminate instances of contamination, mix-ups, and errors for pharmaceuticals or drugs, active pharmaceutical ingredients, diagnostics, foods, and medical devices.
Whilst there is no formal mandatory requirement to comply with cGMP during early clinical development in phase I and phase II Clinical Trials, cGMP MUST be fully implemented by phase III Clinical Trials and for all subsequent manufacturing activities.
A Certification Body applying for GMP accreditation must conform to ISO/IEC 17021 and other additional International requirements as detailed in Specific Requirements for Accreditation for GMP Scheme.

Benefits of Accreditated Certification of GMP Certification
- Businesses perceive accredited certification as providing value for money
- Exceeds minimum standards of quality
- Process and analytical validation
- Providing assurance for the quality
- Achieve International approval
- Deliver value-added outcomes
- Leading independent market research company
- Employers often require evidence that the Certificate that they have received is from an Accredited Body
- Enjoy Competitor Edge
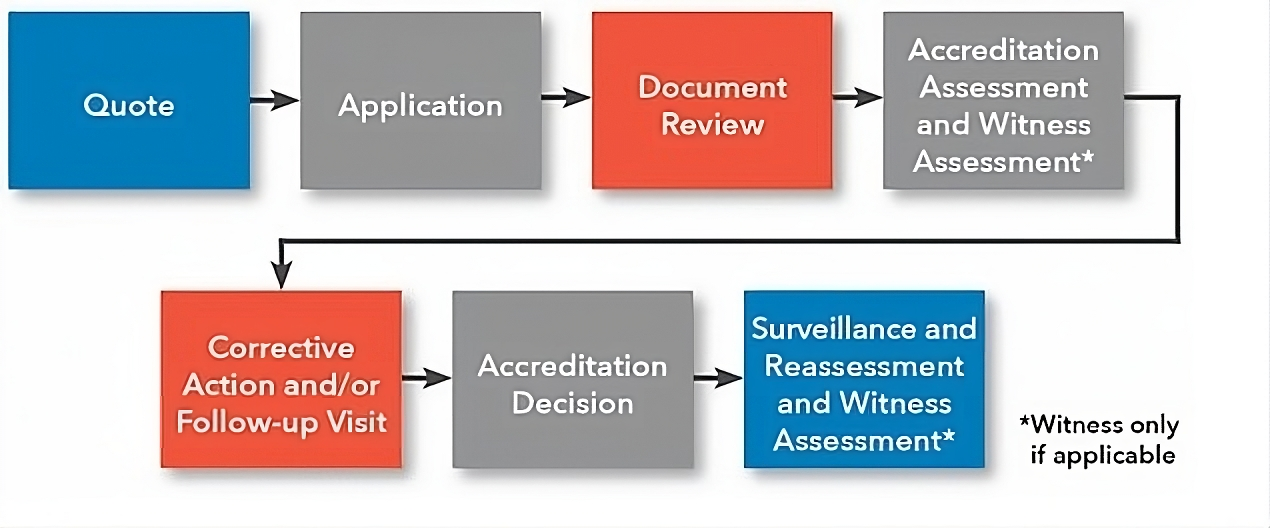
Please refer to the information about the accreditation process
UKAIB Accreditation for Inspection Agencies”
- Demonstrates compliance with GMP Accreditation.
- UKAIB offers prompt, personal service, including rapid scheduling of assessments to meet the needs of inspection agencies.
- Accreditation serves as an internationally recognized “stamp of approval” for industry and regulators.
- Accreditation increases the recognition and acceptance of inspection reports across domestic and national borders.
- Accreditation helps to reduce costs for manufacturers and exporters by reducing or eliminating the need for re-inspecting in another economy.
People Who Love Our Place
UKAIB Accreditation Forum Limited has pioneered novel approaches to accreditation that permit benchmarking via measurable evaluation of conformity assessment body (CAB), performance.




